SAFCell’s fuel cell technology generates electricity through clean, silent electrochemical reactions.
How it works
Fuel cell systems convert chemical energy in fuels into electrical energy. At the core of each fuel cell is a special material called the electrolyte, which allows the production of electricity though efficient electrochemical processes rather than typical fuel combustion. The chemical composition of the electrolyte differentiates one fuel cell technology from another. SAFCell’s technology is based on a new class of electrolytes called solid acids.
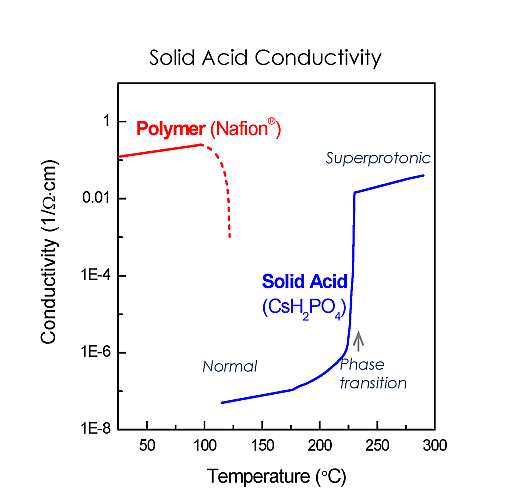
Solid acids are chemical intermediates between normal salts and normal acids. When a normal acid, such as phosphoric acid, reacts with a normal salt, such as cesium carbonate, you end up with cesium dihydrogen phosphate – the current electrolyte in solid acid fuel cells.
At low temperatures, solid acids have ordered structures. However, at warm temperatures, certain solid acids undergo transitions to highly disordered “superprotonic” structures, which causes the conductivity to increase dramatically.
Operating at mid-range temperatures around 250 °C, SAFCell’s stacks tolerate impurities that pose obstacles for other fuel cell technologies, while still using inexpensive metal components and flexible polymer seals.
This allows SAFCell stacks to easily operate on commercially available fuels (e.g. propane, methane, methanol or diesel) while utilizing a very low-cost and rugged platform. SAFCell’s technology enables reduced system complexity and is scalable from tens of watts to tens of kilowatts.