A solid acid fuel cell is a device that silently generates electricity from commercially available fuels.
What is a fuel cell?
A fuel cell is an energy conversion device that converts chemical energy into electrical energy. For example, it can convert a hydrocarbon fuel like propane gas into electricity without combustion.
Think of a fuel cell like a battery - in that it provides continuous DC electricity from a chemical reaction. Just like batteries, a fuel cell has an anode, a cathode, and an electrolyte. Unlike batteries, fuel cells cannot store electrical energy, and they don’t require electricity to charge them. Fuel cells can continuously generate electricity as long as they have a supply of fuel.
Different types of fuel cells
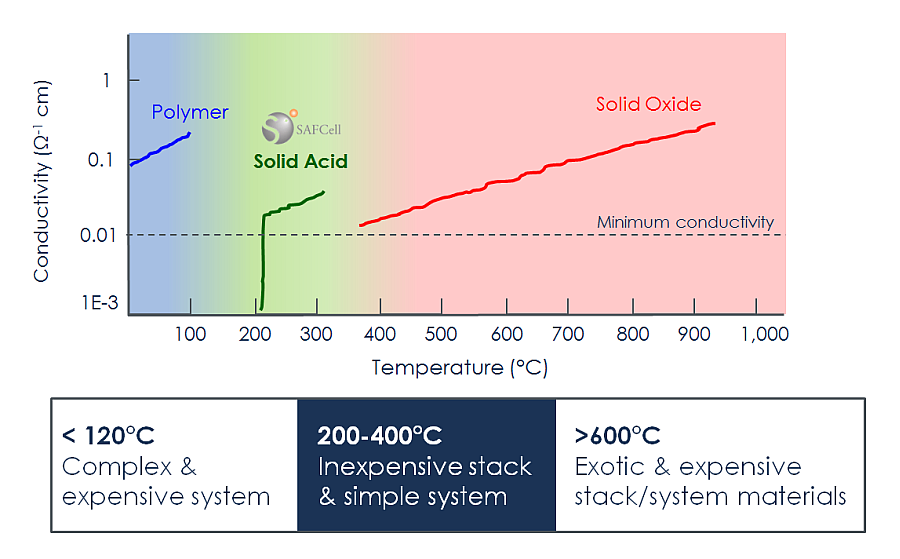
There are five main types of fuel cells. The chemical composition of the electrolyte differentiates one fuel cell technology from another. Different electrolytes transport ions with varying efficiencies as a function of temperature, so that each of these types operates in a different temperature range. SAFCell’s technology is based on a new class of electrolytes called solid acids.
| Fuel Cell Type | Temperature |
|---|---|
| PEMFC - Polymer Electrolyte Membrane | 20-120 °C |
| AFC - Alkaline | 90-120 °C |
| PAFC - Phosphoric Acid | 150-200 °C |
| SAFC - Solid Acid | 220-280 °C |
| MCFC - Molten carbonate | 600-700 °C |
| SOFC - Solid oxide | 700-1000 °C |
Operating at mid-range temperatures around 250 °C, SAFCell’s stacks tolerate impurities that pose obstacles for other fuel cell technologies, while still using inexpensive metal components and flexible polymer seals.
This unique combination of properties results in a rugged and low-cost stack platform that operates easily on commercially available fuels.
Download our fact sheet – What is a Solid Acid Fuel Cell?